Resources
Categories
They are different after all! Atelocollagen, collagen, gelatin, and collagen peptides
What are atelocollagen, collagen, gelatin, and collagen peptides?
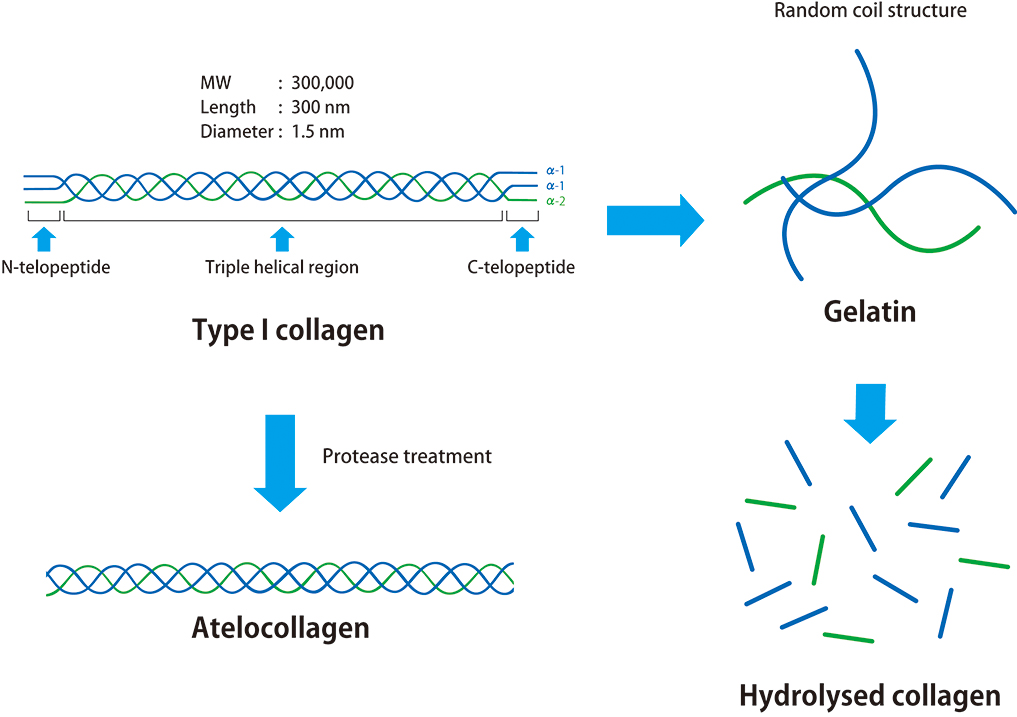
Research into the structural analysis of collagen has progressed since the 1930s, and it has been discovered that type I collagen consists of polypeptide chains with approximately 1,000 amino acids that are bonded together. It is an elongated molecule with three polypeptide chains that form a triple-helix structure. Furthermore, although collagen in animals is an insoluble fibrous protein, it can be extracted using acid.
| Name | General production method | Molecular weight | Gelation |
| Atelocollagen | Enzymatic extraction (low-temperature extraction) | Approximately 300,000 |
Gels when heated (fibrilization) |
| Collagen | Acid extraction (low-temperature extraction) | Approximately 300,000 | Gels when heated (fibrilization) |
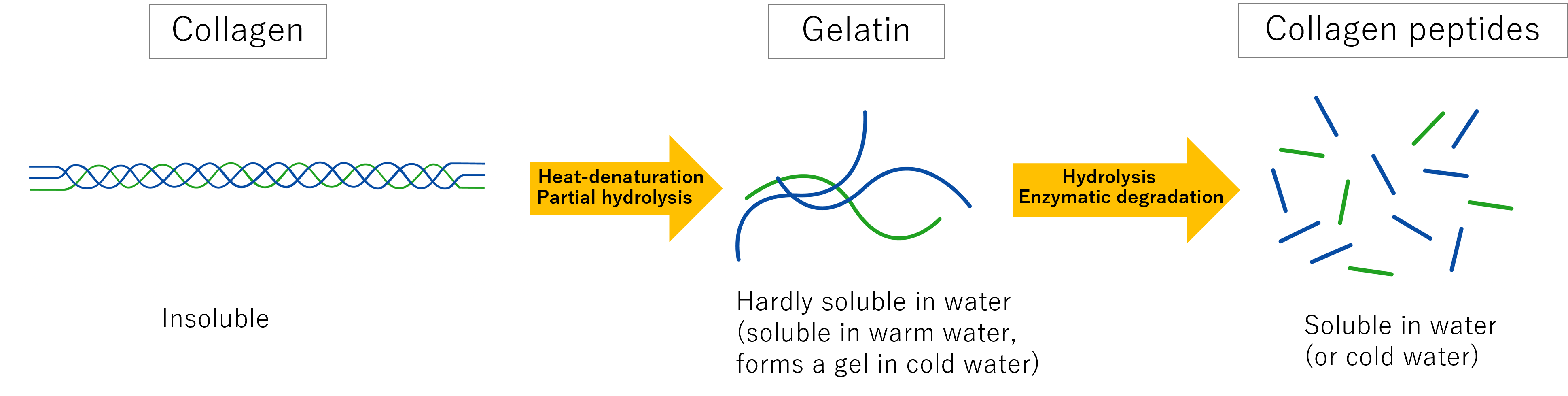
| Gelatin | Acid or alkaline pretreatment and heat treatment | Tens of thousands to hundreds of thousands | Gels when cooled(NOT fibrilization) |
| Collagen peptides | Acid/alkaline/enzymatic degradation | Several hundred to several thousand | Does not gel |
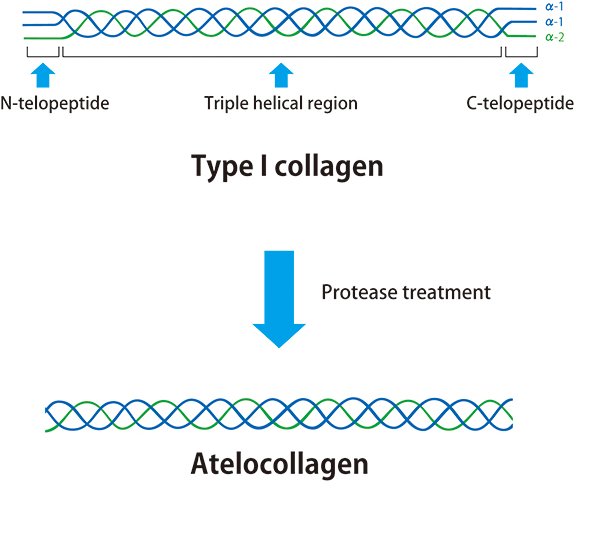
In 1960, a patent application was filed for collagen solubilization technology (patent number: 306922). It used enzyme treatment to remove the antigenic sites (telopeptides at both ends of the collagen molecule), which resulted in highly biocompatible, undenatured collagen = atelocollagen. Since then, atelocollagen-based products have been developed and commercialized. Atelocollagen exhibits properties similar to that of collagen; therefore, under physiological conditions, it undergoes fibrillization wherein the molecules polymerize in an oriented manner, and as fibrillization progresses within a solution, it becomes a visible gel. In addition, because of its low antigenicity, we use it for research reagents as well as for medical devices.
The difference between atelocollagen/collagen and gelatin
The biggest difference between atelocollagen/ collagen and gelatin is the presence or absence of a triple-helix structure. Adhesion with cells occurs through integrin binding to the GxOGER motif, which consists of glycine-hydrophobic amino acids such as phenylalanine–hydroxyproline–glutamic acid–arginine, located on the surface of the atelocollagen/collagen triple-helix structure.
On the other hand, the RGD motif (sequence) consisting of arginine–glycine–aspartic acid, which you have likely heard of, exists in both atelocollagen/collagen and gelatin. However, in the case of atelocollagen/collagen, adhesion with cells through the RGD motif and integrin binding do not occur because they are constrained by the triple-helix molecular structure. Therefore, the interaction of atelocollagen/collagen and gelatin with cells is different.
Furthermore, EDC cross-linking, which is often used to improve the strength of atelocollagen/collagen, forms an amide bond with lysine in the collagen side chain via glutamic acid in the GxOGER motif, and, therefore, it is thought to inhibit the binding between the motif and cells.
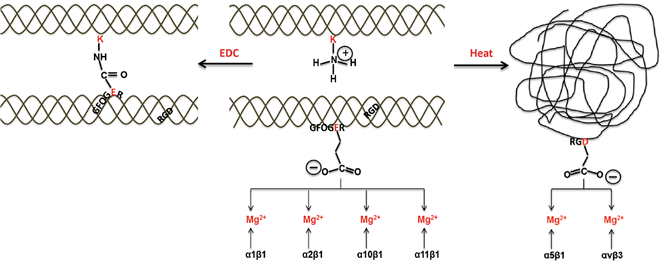
J Mater Sci: Mater Med 27, 148 (2016). Created by cropping figure 1. ©Davidenko N, et al. 2016 (Licensed under CC BY 4.0)
Research using collagen peptides
As mentioned above, collagen peptides have a molecular weight of several hundred to several thousand Daltons, do not undergo fibrilization (gelation), and have properties that are not similar to those of the original collagen. Therefore, unlike collagen, they are not used as a substrate for collagen gel culture. However, many studies have been published on the functions of di- and tri-peptides containing hydroxyproline, an amino acid that is characteristic of collagen. For example, ingesting collagen peptides increases the number of di- and tri-peptides containing hydroxyproline in the blood. In addition, there are reports that the ingestion of collagen peptides has effects such as alleviating arthritis, promoting pressure ulcer healing, and improving skin conditions. Research on collagen peptide functions is expected to continue to progress.
Summary
Substances with significantly different molecular weights and characteristics can be obtained from animal-derived collagen, such as atelocollagen with low antigenicity, gelatin that solidifies when cooled, and collagen peptides that are readily soluble in water. Thus, the interaction of each substance with cells inevitably changes; therefore, if you are unsure of which substance is appropriate for your experiment, please feel free to contact us.