Resources
Categories
Connecting in vitro experiments to in vivo experiments with 3D culture

3D culture is attracting a variety of researchers
Nowadays, many researchers have become interested in 3D culture. Until recently, most visitors to our exhibition booths saw 3D culture as something utilised only in regenerative medicine research. However, in the past few years, cancer researchers have increasingly adopted 3D culture. One of the reasons for this gap between image and reality is the unexpected lack of effective antitumor properties of candidate drugs in animal models, a result that contrasts with the strong antitumor effects observed for these candidates in plate culture. Apparently, standard plate culture does not realistically reflect the actual tumour microenvironment. 3D culture is expected to become more common because researchers are turning to 3D culture in order to perform in vitro experiments under conditions that better mimic in vivo conditions. We have seen increasing numbers of such scientists at exhibitions such as that associated with the annual meeting of the Molecular Biology Society of Japan, where a wide variety of researchers, including those studying regenerative medicine and cancer, have been attending.
3D culture with collagen gel
Collagen accounts for approximately 30% of the total protein in the human body; this protein is widely used for cell culture ware coating due to its high biocompatibility. Actually, the collagen used for coating cell cultureware can fibrillate, such that collagen molecules self-assemble with a specific orientation, eventually forming a gel when the collagen is neutralised and incubated at 37 °C. Utilising the property of gel formation by heating, 3D culture in a collagen gel can be performed with variously shaped cell cultureware by combining a collagen solution with cells and medium and adding the mixture to the cell cultureware
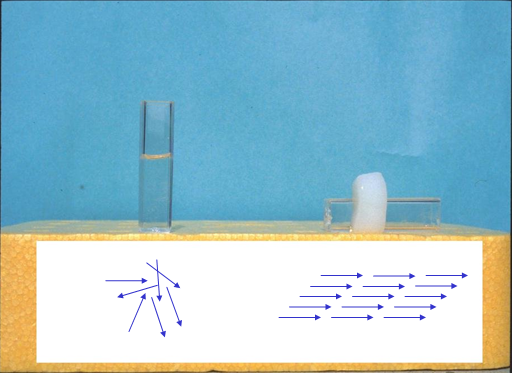
Oxygen supply to cells subjected to 3D culturing in collagen gel
There also is a method for performing 3D culture by forming spheroids. However, as the spheroid diameter increases, cells in the centre of the spheroid tend to die, due in part to impaired exchange and decreased oxygen supply. On the other hand, collagen gel is considered to be capable of permitting 3D culture in an environment better resembling that in the living body. Experiments by Colom et al. showed that when pulmonary epithelial cells were cultured in 0.3% collagen gel for 46 hours, the oxygen partial pressure in the gel reached approximately 20 mmHg; for comparison, the oxygen partial pressure in most tissues such as liver and brain falls into within a range of 20 to 50 mmHg1). Interestingly, in a similar experiment with 0.1% collagen gel, the oxygen partial pressure after 46 hours of culture was about 70 mmHg, a value close to the 75-100 mmHg oxygen partial pressure observed in arteries and alveoli.
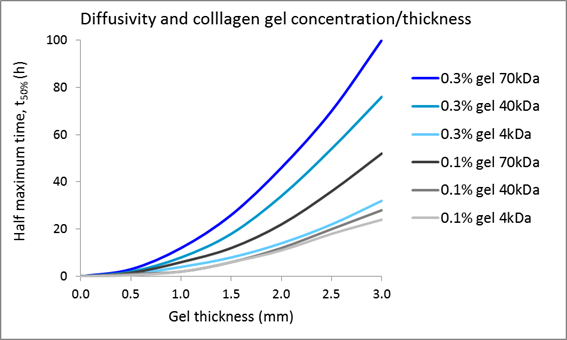
Penetration of physiologically active substance added to collagen gels
While comparative evaluation of cell function by 3D culture and plate culture is the primary interest of many researchers, other scientists are interested in applying 3D culture for experiments using physiologically active substances. When added to the medium in plate culture, those substances are likely to diffuse relatively rapidly. In contrast, the diffusion of such substances is slowed in collagen gels, depending on collagen concentration and thickness. For instance, Galgoczy et al. calculated that the times until 4-kDa, 40-kDa, and 70-kDa substances reach half of the equilibrium concentration in a 2-mm-thick, 0.3% collagen gel containing cells are approximately 16 hours, 34 hours, and 46 hours, respectively2). Those authors proposed also considering whether the physiologically active substance possesses a binding domain for extracellular matrix components such as collagen, and/or adding physiologically active substances from above and below the collagen gel to accelerate the diffusion.
References:
1) Oxygen diffusion and consumption in extracellular matrix gels: Implications for designing three-dimensional cultures.
Colom A, Galgoczy R, Almendros I, Xaubet A, Farré R, Alcaraz J.J Biomed Mater Res A. 2014 Aug;102(8):2776-84. PMID: 24027235
2) A spectrophotometer-based diffusivity assay reveals that diffusion hindrance of small molecules in extracellular matrix gels used in 3D cultures is dominated by viscous effects.
Galgoczy R, Pastor I, Colom A, Giménez A, Mas F, Alcaraz J.
Colloids Surf B Biointerfaces. 2014 Aug 1;120:200-7. PMID: 24916283