Resources
Categories
Oligonucleotide therapy development around the world

Revival of oligonucleotide therapy development
At one point, many pharmaceutical companies were conducting research on oligonucleotide therapy, which employs chemically synthesized nucleotide-like small molecule drugs with potential specificity similar to that of therapeutic antibodies. However, the development of oligonucleotide therapy has since slowed, with some major pharmaceutical companies terminating the relevant research programs completely. One of the reasons is thought to be the immature state of the drug delivery system (DDS) that would be required for transporting nucleic acids to the target site. Nevertheless, the development of oligonucleotide therapy has again begun to draw attention along with research progress on oligonucleotide therapy without DDS, which has been achieved by increasing these drugs’ stability in the body by chemical modification of the nucleic acid-like molecules.
Types of oligonucleotide therapies
Although several types of oligonucleotide therapies exist, five of them are compared here, including the ones targeting nucleic acids (such as mRNA) or proteins, as well as others intended for stimulation of the immune system. DDS is generally used with oligonucleotide therapy, as it is difficult for these molecules to penetrate cell membrane when administered on their own.
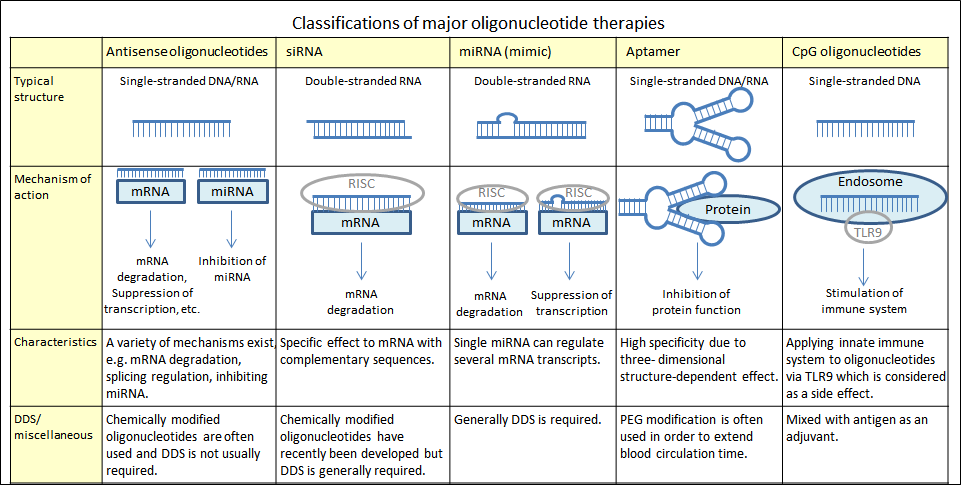
Approved/late-stage development oligonucleotide therapies
As shown in the following list, seven products have been approved as of January 2018; most of these products are antisense oligonucleotides, and almost all have been chemically modified to enhance their stability in the body. Earlier approved products were limited to local injection into affected tissues, but subcutaneous and intravenous administration products are now on the market. It should be noted that Defitelio is not a synthetic oligonucleotide per se, but instead a polydispersed mixture of single-stranded oligonucleotides derived from porcine intestinal tissue, which is why this product is designated as “miscellaneous” here.
| Approved oligonucleotide therapies | ||||||
|---|---|---|---|---|---|---|
| Company | Trade name | INN | Type | Indication | Modification | Administration |
|
Novartis [Co-developed with Ionis Pharmaceuticals (formaly Isis Pharmaceuticals)] |
Vitravene 【withdrawn】 |
fomivirsen | Antisense oligonucleotide (ODN) |
CMV retinitis in patients with AIDS | S-oligo | Intravitreal injection |
| Valeant Pharmaceuticals (formaly EyeTech Pharmaceutical) [Co-developed with Pfizer] |
Macugen | pegaptanib | Aptamer | neovascular (wet) age-related macular degeneration | 5′-pegylated 2′-F 2′-OMe |
Intravitreal injection |
| Kastle Therapeutics [Developed by Ionis Pharmaceuticals (formaly Isis Pharmaceuticals) and Sanofi Genzyme (formaly Genzyme)] |
Kynamro | mipomersen | Antisense ODN | Homozygous familial hypercholesterolemia | S-oligo 2′-MOE |
Subcutaneous injection |
| Sarepta Therapeutics | Exondys 51 | eteplirsen | Antisense ODN | Duchenne muscular dystrophy | Phosphorodiamidate morpholino oligomer | Intravenous injection |
| Biogen [Co-developed with Ionis Pharmaceuticals (formaly Isis Pharmaceuticals)] |
Spinraza | nusinersen | Antisense ODN | Spinal muscular atrophy | S-oligo 2′-MOE |
Intrathecal injection |
| Dynavax Technologies | Heplisav-B | Hepatitis B Vaccine (Recombinant) Adjuvanted |
CpG ODN | Hepatitis B | S-oligo | Intramuscular injection |
| Miscellaneous | ||||||
| Jazz Pharmaceuticals (formaly Gentium) | Defitelio | Defibrotide | ODN derived from porcine intestinal tissue | Hepatic veno-occlusive disease | – | Intravenous injection |
Note: This list was produced on the basis of publicly available information; it may not reflect the most recent developments.
Given that only seven products have been approved, oligonucleotide therapies may appear to still be under development. However, there are another five products at the stage of approval review, 12 products in Phase III trials, and one cosmetic ingredient at the consumer test stage. According to data from the Japan patent office, 141 clinical trials with oligonucleotide therapies were being conducted as of October 2015. Given that nearly 20 products are approaching commercialisation and that the number of siRNA products is increasing, we can expect a variety of oligonucleotide therapies to be available in the near future.
| Major late-stage development oligonucleotide therapies | ||||
|---|---|---|---|---|
| Company | Name | Type | Indication | Stage |
| Alnylam | Patisiran | siRNA | Hereditary amyloidosis | Approval review |
| Inclisiran | siRNA | Hypercholesterolemia | P3 | |
| Fitusiran | siRNA | Hemophilia and rare bleeding disorders | P3 | |
| Givosiran | siRNA | Acute hepatic porphyrias | P3 | |
| Gene Signal | Aganirsen | Antisense ODN | Neovascular-associated corneal graft rejection | P3 |
| Aganirsen | Antisense ODN | Ischemic central retinal vein occulusion | P3 | |
| Geron | Imetelstat | lipid-conjugated ODN | Myelodysplastic syndromes | P3 |
| Ionis Pharmaceuticals | Alicaforsen | Antisense ODN | Pouchitis | Approval review |
| Inotersen | Antisense ODN | TTR amyloidosis | Approval review | |
| Volanesorsen | Antisense ODN | Familial chylomicronemia syndrome | Approval review | |
| Volanesorsen | Antisense ODN | Familial partial lipodystrophy | Approval review | |
| Achive Life Sciences | Custirsen | Antisense ODN | Non-small cell lung cancer | P3 |
| Quark Pharmaceuticals | QPI-1002 | siRNA | Delayed graft function | P3 |
| QPI-1007 | siRNA | Non-arteric anterior ischemic neuropathy | P3 | |
| Sarepta Therapeutics | SRP-4045 | Antisense ODN | Duchenne muscular dystrophy | P3 |
| SRP-4053 | Antisense ODN | Duchenne muscular dystrophy | P3 | |
| Sylentis | Tivanisiran | siRNA | Dry eye syndrome | P3 |
| Miscellaneous | ||||
| RXi Pharmaceuticals | RXI-23 | siRNA | Cosmetic ingredient | Consumer test |
Note: This list was produced on the basis of publicly available information; it may not reflect the most recent developments.
DDS research for oligonucleotide therapy
Neither of the currently approved oligonucleotide therapies uses DDS. Kynamro, which is administered subcutaneously, takes advantage of a characteristic property of oligonucleotide, namely the tendency to accumulate in the liver and kidney. Exondys 51, which is administrated intravenously, utilises the tendency toward increased uptake by muscle cells in patients with Duchenne muscular dystrophy. However, those advantages are not always available in other target tissues and/or diseases; a number of studies have investigated the effective delivery of nucleic acids using DDS. For example, PEGylated liposomes are well known for their longer blood circulation time, and vitamin A-modified liposomes have been used to target hepatic stellate cells. Last but not least, atelocollagen-based DDS has been developed. Unlike liposome-based DDS, atelocollagen has no toxicity and can facilitate permeation of blood vessels when complexed with nucleic acids.

